In a pioneering study, stem-cell-derived corneal implants improve sight for patients with severe vision impairment, offering a potential breakthrough for treating limbal stem cell deficiency.
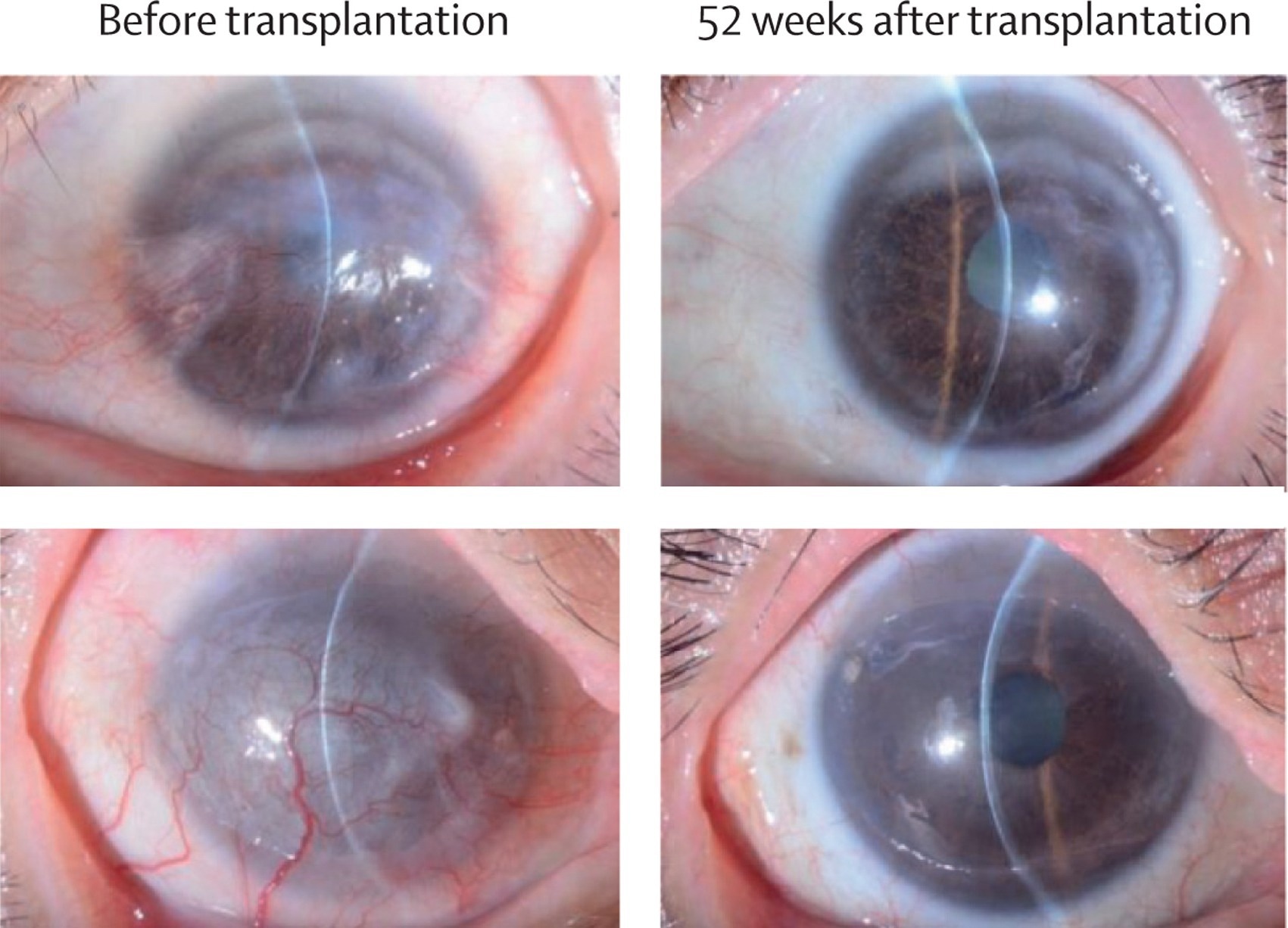
Slit-lamp microscopic photographs of all four treated eyes before and 52 weeks after induced pluripotent stem cell-derived corneal epithelial cell sheet transplantation. Study: Induced pluripotent stem-cell-derived corneal epithelium for transplant surgery: a single-arm, open-label, first-in-human interventional study in Japan
In a recent study published in The Lancet, a group of researchers evaluated the safety and preliminary efficacy of human induced pluripotent stem cell (iPSC)-derived corneal epithelial cell sheets (iCEPS) for treating limbal stem cell deficiency (LSCD), a debilitating condition characterized by the loss of corneal stem cells, which causes severe vision impairment.
Background
The corneal epithelium, essential for vision, relies on limbal stem cells located at the corneal edge for continuous regeneration. LSCD occurs when these cells are lost or depleted, leading to damage to the corneal surface, conjunctival scarring, and vision impairment. LSCD can result from trauma, immune-mediated conditions, or genetic disorders. Treatment involves reconstructing the ocular surface and grafting healthy corneal epithelial tissue. Both autologous and allogeneic therapies face limitations like immune rejection risks, biopsy dependence, and quality inconsistencies. iPSC-derived corneal grafts offer promise, though further research is essential to validate efficacy and safety on a larger scale.
About the Study
In the present study, conducted at Osaka University Hospital’s Department of Ophthalmology, four participants with LSCD were enrolled and observed for 52 weeks, with an additional 52-week safety monitoring period. The study complied with Japan’s regulatory standards for regenerative medicine and was approved by relevant health and ethics committees. All participants were adults with LSCD stages ranging from moderate stem cell loss with noticeable vision impact (IIB) to advanced loss with extensive corneal damage (IIC) or complete stem cell loss with severe vision impairment (III). Informed consent was obtained after comprehensive explanations using accessible materials for visually impaired patients.
Two patients received iCEPS mismatched for Human Leukocyte Antigen (HLA) type. Six months after transplant, their response determined the HLA compatibility and immunosuppressive requirements for the following two transplants. Exclusion criteria included allergies, recent malignancies, uncontrolled diabetes, infectious diseases, and certain medication uses. Pregnant or lactating women were also excluded. Patients were recruited through institutional networks, meeting strict inclusion criteria.
The procedures included creating and purifying corneal stem cells from iPSCs, manufacturing iCEPS, and performing a specialized keratectomy to remove fibrotic tissue. The iCEPS were then placed on the patient’s eyes and secured. Postoperative care involved antibiotics, corticosteroids, and therapeutic contact lenses. Safety served as the primary endpoint, with adverse events monitored and classified throughout; efficacy metrics such as visual acuity, LSCD stage, and corneal health were assessed preoperatively and at several postoperative intervals.
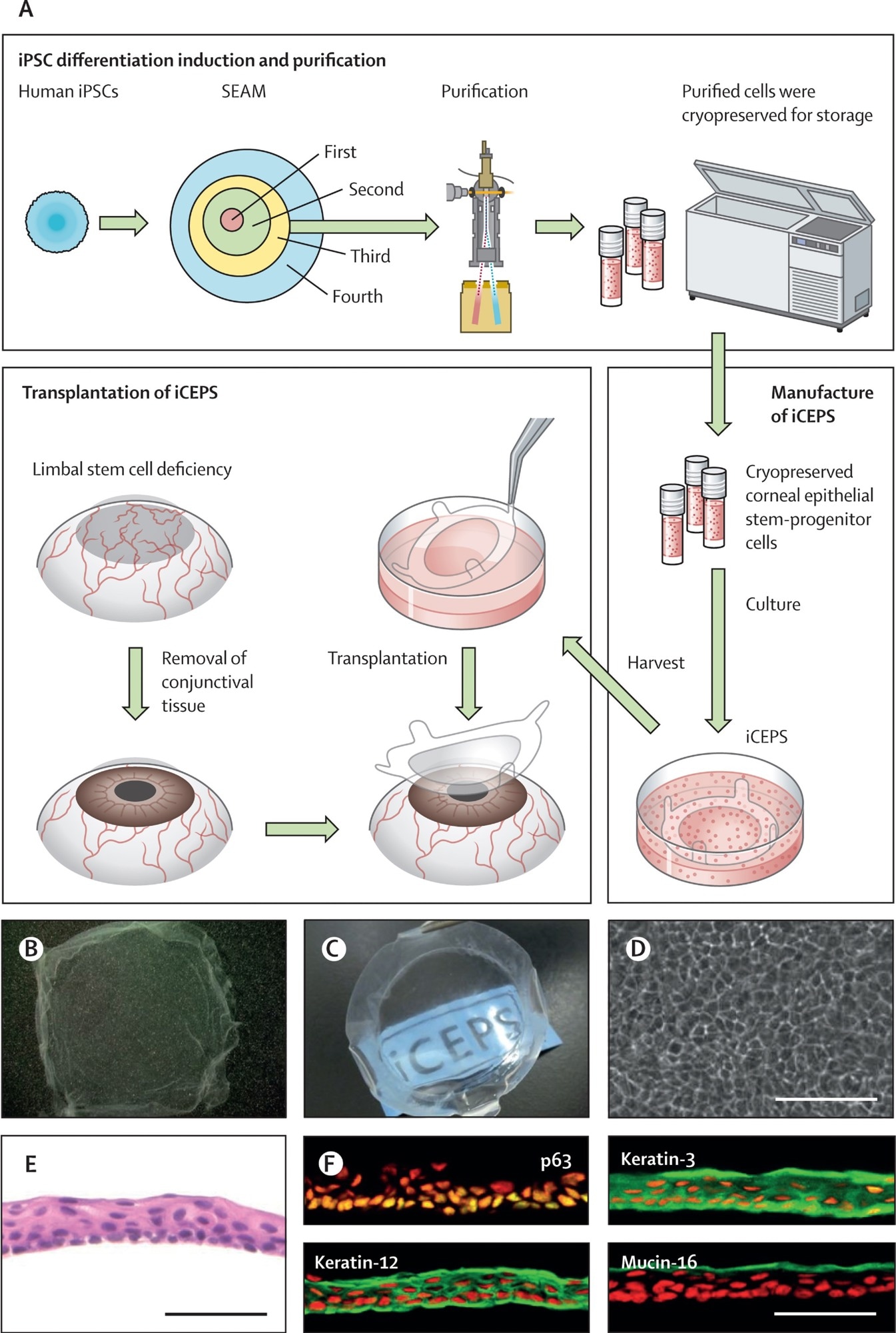
(A) A schematic of the whole procedure, starting with the cultivation of human iPSCs into SEAMs and the subsequent purification of corneal epithelial stem–progenitor cells from the third zone of the SEAM by means of a cell sorter. These cells were cryopreserved before their fabrication into iCEPSs at a good gene, cellular, and tissue-based products manufacturing practice-grade facility, and the subsequent transplantation of iCEPS onto affected eyes after removal of conjunctival tissue in patients with an LSCD. (B, C) Macrophotographs of iCEPSs before engraftment showing their transparent nature, with the text “iCEPS” visible below the construct. (D) Cobblestone-like appearance of cells in an iCEPS imaged by phase contrast microscopy. Scale bar, 100 μm. (E) iCEPSs are made up of three to five cell layers and resemble the normal corneal epithelium on haematoxylin and eosin staining. Scale bar, 50 μm. (F) iCEPSs are immunostained green and positive for p63, keratin-12, keratin-3, and mucin-16, identifiers of the corneal epithelium; the nuclei are shown in red. Scale bar, 50 μm. iPSC=induced pluripotent stem cell. iCEPSs=iPSC-derived corneal epithelial cell sheets. LSCD=limbal stem cell deficiency. SEAM=self-formed, ectodermal, autonomous, multi-zone.
Study Results
The iCEPS used in this study were transparent and consisted of cells with a characteristic cobblestone appearance. These cells formed a multilayer structure and expressed key markers of corneal epithelium, including tumor protein 63 (p63), keratin-12, keratin-3, and mucin-16. Rigorous tumorigenicity assessments, including tests in immunodeficient mice and karyotype analyses, confirmed no tumorigenic potential. Each iCEPS sheet underwent thorough quality control checks before transplantation, ensuring compliance with established standards.
Participants, recruited between June 2019 and November 2020, included four patients with LSCD. The first two patients were a 44-year-old female with idiopathic LSCD and a 66-year-old male with ocular mucous membrane pemphigoid. These patients received HLA-mismatched iCEPS transplants and underwent mid-term evaluations revealing no signs of immunological rejection. Consequently, the research team concluded that additional immunosuppression and HLA compatibility were not needed for the subsequent two patients, a 72-year-old male with idiopathic LSCD and a 39-year-old female with toxic epidermal necrosis.
No cases of immunological rejection or tumor formation were observed throughout the study, and no serious adverse events occurred. A total of 26 minor adverse events were recorded across the four patients over the 52-week follow-up, all of which were mild and manageable. Nine non-serious adverse events were documented during the additional one-year safety monitoring period.
Clinical improvements were observed in all patients. LSCD severity decreased, with patients 1 and 2 improving from stage III to IA, and patient 3 from stage IIB to IA, maintaining this improvement throughout the study. Patient 4, with the most severe baseline condition, initially showed improvement to stage IA but regressed to stage IIB after one year. Corneal epithelial defects were either stable or improved, with patients 1, 2, and 3 achieving grade 0 (no defect) by week 52, while patient 4 remained at grade 1.
All patients reported either stabilized or improved symptoms. Corrected distance visual acuity also improved, most notably for patients 1 and 2, and all patients exhibited a reduction in corneal opacification. Quality of life scores increased for patients 1, 2, and 3, though patient 4 experienced a decline. Corneal neovascularization decreased for patients 1 and 2 but remained stable or increased in patients 3 and 4. Symblepharon severity was unchanged in all cases by week 52.
Conclusions
To summarize, in this first-in-human study, four patients tolerated the transplants without tumor formation or immunological rejection, supporting the procedure’s safety. Improvements in LSCD stage and other efficacy markers were observed in most cases, especially without HLA matching, suggesting iCEPS as a potentially viable option. Pre-transplant tests confirmed the absence of tumorigenic risks, reinforcing the safety profile. Despite some observed regression in patient 4, likely due to subclinical immune responses, patients 1 and 2 experienced substantial improvements.
Future Directions
This study provides groundbreaking evidence for iPSC-derived corneal epithelial cell transplants in treating LSCD and suggests potential advantages over traditional methods. Further multicenter clinical trials are planned to expand on these results and confirm the broader efficacy of iCEPS transplantation as a new therapeutic option for LSCD patients.