New study identifies insulin ‘chain-splitting’ during bloodstream transit as a game-changer in understanding and treating diabetes and insulin resistance.
 Study: Chain splitting of insulin: an underlying mechanism of insulin resistance? Image Credit: Nastasiiaa / Shutterstock
Study: Chain splitting of insulin: an underlying mechanism of insulin resistance? Image Credit: Nastasiiaa / Shutterstock
In a recent study published in the journal NPJ Metabolic Health and Disease, researchers hypothesize that the degradation of endogenously secreted insulin (as opposed to the commonly believed insulin receptor signaling defects) is the mechanism underpinning insulin resistance in humans. The hypothesis posits that thiol-mediated ‘chain-splitting,’ which depends on the redox potential of the plasma environment, occurs when human insulin (HI) is degraded by redox reactions at concentrations typical for human plasma.
They substantiate their chain-splitting hypothesis with fresh evidence from both in vitro (human plasma) and in vivo (rats infused with human insulin) and demonstrate that the degradation of A- and B- HI chains results in lowered insulin availability at target cells, thereby directly contributing to observed insulin resistance. Notably, the study highlights that chain-splitting rates align with the redox potentials typically found in human plasma, supporting the physiological relevance of the findings. These findings challenge current worldviews on the mechanism governing insulin resistance and provide a novel research avenue for future pharmacological interventions against the condition.
Background
Insulin resistance is a chronic and severe medical condition that occurs when the body’s cells do not adequately respond to circulating endogenous insulin. Since insulin is the hormone governing glucose uptake, insulin resistance often results in progressively increasing blood glucose levels, substantially increased prediabetes and type 2 diabetes (T2D) risk, in turn contributing to obesity, cardiovascular diseases (CVDs), metabolic syndrome, and polycystic ovary syndrome (PCOS).
Furthermore, insulin resistance (specifically, spikes in blood glucose levels) forces the pancreas to compensate through increased insulin production and secretion. The persistent inability of cells to respond to this increased secretion triggers a positive feedback loop, eventually contributing to pancreatic diseases or failure. Together, these findings highlight the need for an enhanced understanding of the mechanisms governing insulin resistance, allowing for pharmacological interventions against this condition that are estimated to impact between 15.5% and 46.5% of all adults.
Unfortunately, despite decades of research, the cascade of events resulting in insulin-resistant phenotypes remains poorly understood. Current worldviews recognize the multifactorial nature of insulin resistance but assume that target tissue/cell defects or insulin receptor signaling inadequacies govern observed insulin resistance. Emerging evidence suggests that plasma redox states, influenced by factors such as diet, lifestyle, and exercise, may modulate insulin degradation mechanisms, adding complexity to this model.
About the Study
In the present study, researchers hypothesize a novel mechanism of insulin resistance termed ‘chain-splitting.’ The hypothesis posits that the degradation of endogenous insulin during its journey from the pancreas to target cells, not defects in the cells themselves, results in insulin-resistant phenotypes. This hypothesis underscores the role of redox potentials in the plasma environment in driving the chain-splitting process. They use in vitro and in vivo experiments to demonstrate the chain-splitting process across A—and B—insulin chains and substantiate their claims with data from the literature.
Study data was obtained from two healthy human volunteers (in vitro experiments) and male Sprague Dawley rats (~350g; in vivo). Experimental procedures began with the isolation of human insulin (HI) from the blood plasma of the human participants. Purified HI was treated with a glutathione redox couple comprising reduced (GSH) and oxidized forms (GSSG), initiating chain-splitting in the HI A-chain. Lower redox potentials were found to accelerate chain-splitting, reinforcing the significance of redox conditions in insulin degradation. The resultant A-chain was purified using a Reversed-phase high-performance liquid chromatography (RP-HPLC) column.
For in vivo experiments, rats fasted overnight were infused with purified HI at two nmol/kg/min alongside constant monitoring (every 10 minutes) and adjustments to glucose infusion rates (GIR). Blood samples collected at 10, 20, 30, 60, 120, and 180 min were used to quantify insulin and free A-/B-chain concentrations.
All experimental data was acquired via liquid chromatography-mass spectroscopy (LC-MS) systems (TLX-2 TurboFlow high-performance LC system and Acquity I-Class LC system for plasma stability analysis and HI/B-chain quantification, respectively). Nonlinear least squares carried out in GraphPad Prism 9.0.1 were used for statistical analyses of obtained data.
Study Findings
The study demonstrates that a substantial portion of HI suffers degradation via A- and B-chain splitting during transit from the pancreas to the target cells. While this phenomenon has been predicted in previous research, its impact was assumed to be negligible, in contrast to current study findings. The GSH/GSSG (redox) couple was found to play a significant role in HI degradation, with lower redox potentials increasing the rate of HI chain-splitting.
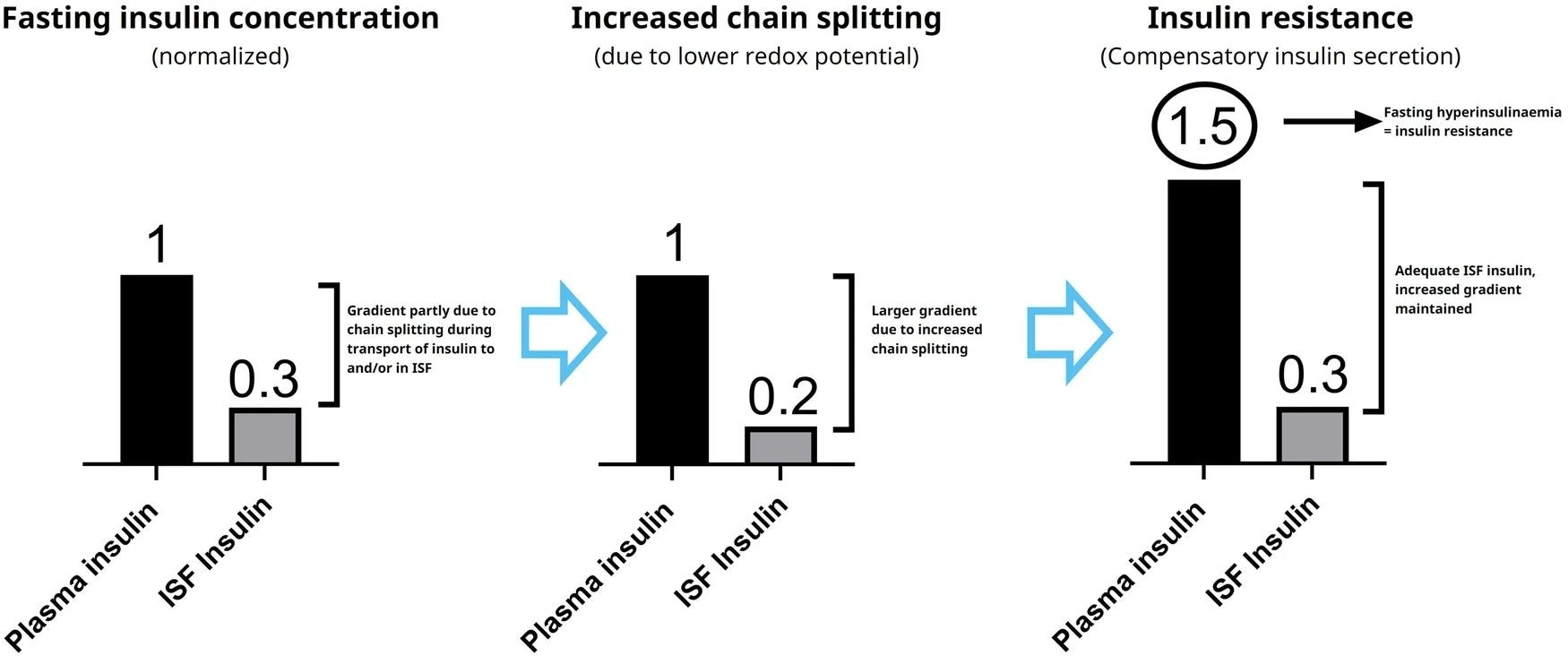
The left panel represents the insulin concentration gradient from published data18 in healthy individuals. The middle panel illustrates how increased chain splitting will result in a larger gradient according to our hypothesis, leading to compensatory insulin secretion, plasma hyperinsulinaemia and thus insulin resistance as shown in the right panel.
Notably, the GSH/GSSG redox potentials required for chain-splitting match normal endogenous levels in human blood plasma, supporting the physiological validity of these findings. Additionally, plasma redox states influenced by factors such as diet and exercise may modulate the rate of insulin chain-splitting, potentially altering insulin sensitivity. The current hypothesis is further substantiated by in vivo experiments, wherein rats infused with HI mirrored in vitro blood plasma observations.
“Based on the plasma A-chain levels in the clamp study and on the A-chain clearance kinetics determined in the separate PK experiment, we estimate that the A-chain appearance rate (i.e., the rate of HI chain splitting) in the clamp study corresponds to 0.40 nmol/kg/min or approximately 20% of the HI infusion rate, clearly demonstrating that chain splitting is an in vivo relevant degradation mechanism also for HI.”
Conclusions
The present study provides evidence in support of a novel mechanism of insulin resistance, which posits that in-transit insulin degradation (‘chain-splitting’) is an underlying determinant of insulin-resistant phenotypes. This alternative hypothesis deviates from current insulin resistance worldviews, the latter of which assume that defects in target tissues or cells prevent normal insulin uptake. Furthermore, the findings suggest that factors like diet, exercise, and redox state manipulation may influence the degradation of insulin, opening avenues for integrative treatment approaches. These findings merit further research and may present the first step in a new class of pharmacological interventions against human insulin resistance and its comorbidities.
Journal reference:
- Cramer, C. N., Hubálek, F., Brand, C. L., Helleberg, H., Kurtzhals, P., & Sturis, J. (2024). Chain splitting of insulin: An underlying mechanism of insulin resistance? Npj Metabolic Health and Disease, 2(1), 1-6. DOI: 10.1038/s44324-024-00042-1, https://www.nature.com/articles/s44324-024-00042-1