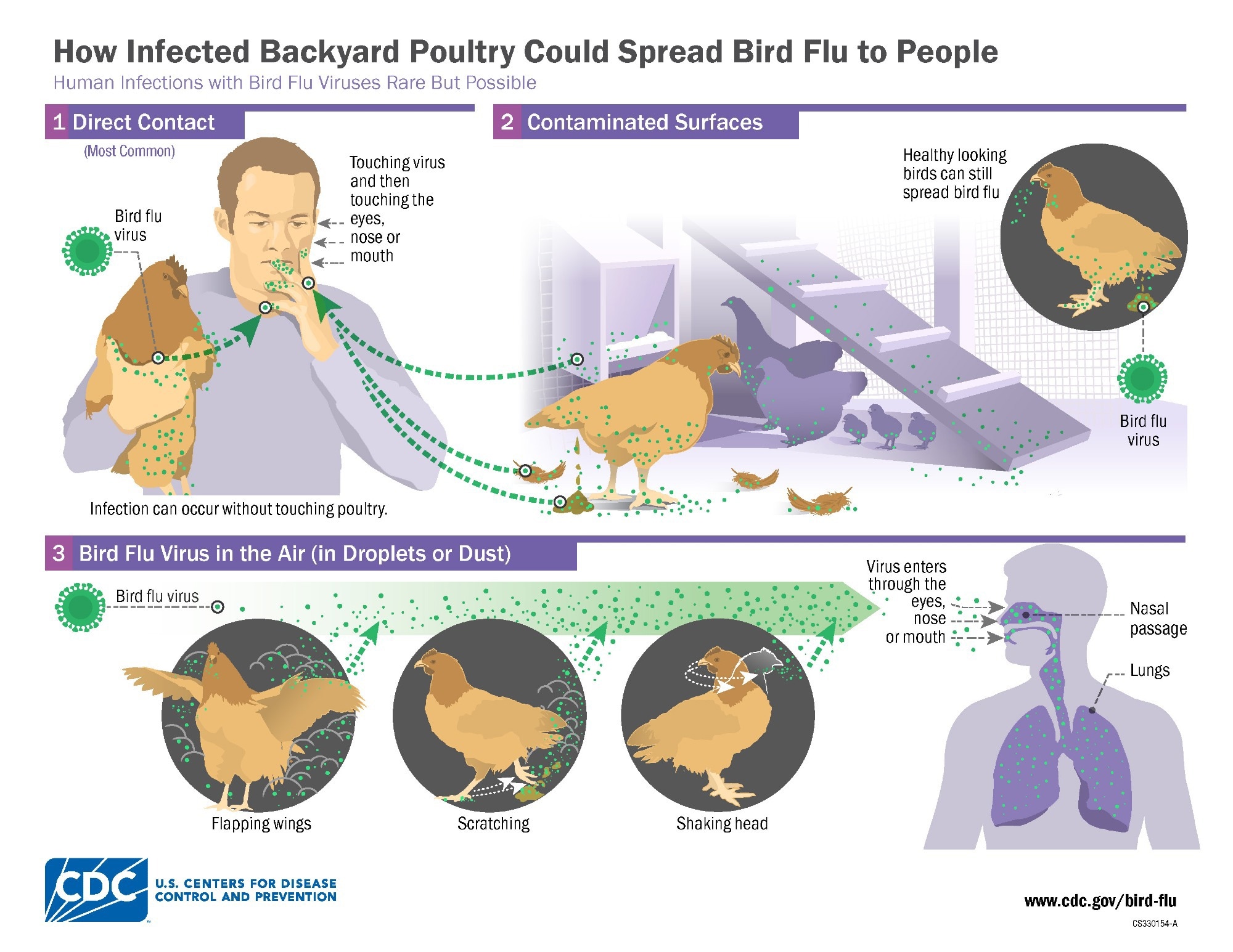
Researchers uncover how a key human immune protein curbs H5N1 avian flu, but the virus is evolving—could we be on the brink of a new zoonotic threat?
 Research Letter: Replication Restriction of Influenza A(H5N1) Clade 2.3.4.4b Viruses by Human Immune Factor, 2023–2024. Image Credit: CDC
Research Letter: Replication Restriction of Influenza A(H5N1) Clade 2.3.4.4b Viruses by Human Immune Factor, 2023–2024. Image Credit: CDC
A recent study published in the journal Emerging Infectious Diseases showed that the human immune factor, myxovirus resistance protein 1 (MxA), represses the replication of highly pathogenic avian influenza A (HPAI) viruses of the H5N1 subtype.
HPAI H5N1 clade 2.3.4.4b viruses have increasingly caused mammalian outbreaks over the past few years. In the United States (US), outbreaks have been detected in cows since Spring 2024, leading to viral transmission to farm workers, possibly via contact with contaminated milk or infected cows. This has sparked concerns that these viruses may adapt to humans.
Notably, some mammalian H5N1 clade 2.3.4.4b isolates already harbor mutations that enhance viral polymerase activity in mammalian cells, binding to mammalian receptors, or evasion from the BTN3A3 restriction factor. Specific mutations, such as PB2E627K and PB2M631L within the PB2 627 domain, are known to contribute to these adaptations. MxA is an innate immune protein that can repress the replication of zoonotic influenza A viruses (IAVs).
Studies have reported that human-adapted IAVs, including the pandemic H1N1 virus A/Hamburg/4/2009 (pH1N1), escape MxA restriction through adaptive amino acids. However, avian IAVs, including the H5N1 clade 2.3.4.4b isolates and human HPAI H5N1 isolate A/Thailand/1(KAN-1)/2004, lack these MxA evasion-mediating amino acids.
The study and findings
The present study investigated whether MxA restricts zoonotic infections with mammalian H5N1 clade 2.3.4.4b isolates. First, the researchers examined MxA’s antiviral activity against blue fox H5N1 and white mink H5N1 viruses isolated from Finnish fur farms, cat H5N1 virus from Poland, and bovine H5N1 from the US.
The prototypical HPAI H5N1 KAN-1 and human pH1N1 viruses were used as controls. Growth of all H5N1 2.3.4.4b isolates and KAN-1 was repressed in active MxA-overexpressing MDCK (MDCK-MxA) cells. By contrast, viral replication reached peak titers in cells with inactive MxA. pH1N1 viral replication was slightly lower in MDCK-MxA cells.
Next, C57BL/6 (B6) mice lacking a functional Mx protein and transgenic mice expressing human MxA were intranasally inoculated with KAN-1, pH1N1, or mammalian H5N1 clade 2.3.4.4b isolates. pH1N1 had comparable lung titers in transgenic and B6 mice three days post-infection, while KAN-1 replication was more than 3000-fold lower in transgenic animals than in B6 mice.
Notably, viral replication of clade 2.3.4.4b isolates declined by up to 100-fold in transgenic mice. Besides, transgenic mice were clinically healthy with no sign of disease, while B6 mice showed lethargy, hunched posture, and ruffled fur. Prior studies have reported that some pandemic IAVs overcame MxA restriction through the reassortment of avian IAV proteins with mammal-adapted viral polymerase components or nucleoprotein (NP).
As such, the team evaluated whether replacing NP or polymerase components would render the H5N1 HPAI polymerase complex MxA-resistant. They observed robust suppression of viral polymerase activity when the bovine H5N1 polymerase complex was reconstituted with bovine H5N1 NP in the presence of MxA. By contrast, MxA’s inhibitory effect was not observed after reconstituting bovine H5N1 polymerase with pH1N1 NP. Interestingly, partial evasion of MxA restriction was achieved through substitutions in the human-adapted polymerase acidic (PA) component, whereas polymerase basic 1 (PB1) or PB2 components did not exhibit this effect.
Finally, the team assessed the antiviral activity of swine, ferret, and cow Mx1 proteins against different viral polymerase complexes, highlighting the role of host-specific Mx1 proteins in viral replication dynamics. They observed the suppressive effect of bovine and swine Mx1 proteins against HPAI H5N1 polymerase but not against pH1N1 polymerase. Ferret Mx1 lacked antiviral activity, emphasizing the variability in Mx1 functionality across species.
Conclusions
The findings indicate that human MxA restricts prevailing mammalian H5N1 clade 2.3.4.4b isolates. Nevertheless, this restriction was less pronounced in transgenic mice, suggesting that adaptations in the viral polymerase may have enabled partial evasion of MxA restriction. Enhanced surveillance focusing on both mammalian outbreaks and host-specific Mx1 activity could help identify potential MxA-evasive variants early, providing critical warnings for public health.