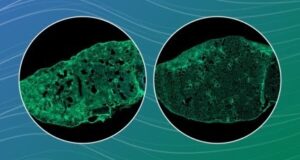
The secret to cellular youth may depend on keeping the nucleolus—a condensed structure inside the nucleus of a cell—small, according to Weill Cornell Medicine investigators. The findings were elucidated in yeast, a model organism famous for making bread and beer and yet surprisingly similar to humans on the cellular level.
The study, published Nov. 25 in Nature Aging, may lead to new longevity treatments that could extend human lifespan. It also establishes a mortality timer that reveals how long a cell has left before it dies.
As people get older, they are more likely to develop health conditions, such as cancer, cardiovascular disease and neurodegenerative diseases.
“Aging is the highest risk factor for these diseases,” said Dr. Jessica Tyler, professor of pathology and laboratory medicine at Weill Cornell Medicine. “Rather than treating each disease separately, a better approach would be to develop a therapeutic or supplement that will delay the onset of diseases by preventing the underlying molecular defects that cause them.” The nucleolus may hold the key.
Small packages
The nucleus holds the cell’s chromosomes and the nucleolus where the ribosomal DNA (rDNA) is housed. The nucleolus isolates the rDNA which encodes the RNA portions of the ribosomes, the protein-building machinery. The rDNA is one of the most fragile parts of the genome, due to its repetitive nature making it more difficult to maintain and fix if damaged. If damage in the rDNA is not accurately repaired, it can lead to chromosomal rearrangements and cell death.
In organisms from yeast to worms to humans, nucleoli expand during aging. On the flip side, anti-aging strategies like calorie restriction, or eating less, result in smaller nucleoli. “Calorie restriction does so many different things, and no one knows the precise way that it is extending lifespan,” Dr. Tyler said.
Dr. Tyler and postdoctoral fellow Dr. J. Ignacio Gutierrez, the first author of the paper, suspected that keeping nucleoli small could delay aging. To test this idea, they engineered an artificial way to secure rDNA to the membrane surrounding the nucleus of yeast cells so they could control when it was anchored and when it was not.
The advantage of our system is that we could isolate the nucleolus size from all of the other effects of anti-aging strategies.”
Dr. J. Ignacio Gutierrez, postdoctoral fellow
The researchers discovered that tethering the nucleolus was enough to keep it compact, and small nucleoli delayed aging to about the same extent as calorie restriction.
Final moments
Interestingly, nucleoli did not expand at the same rate during the entire lifespan as cells aged. They remained small for most of the yeast’s life, but at a nucleolar size threshold, the nucleoli suddenly began to grow quickly and expand to a much larger size. Cells only survived for an average of about five more cell divisions after hitting this threshold.
“When we saw it wasn’t a linear size increase, we knew something really important was happening,” said Dr. Gutierrez. Passing the threshold appears to serve as a mortality timer, ticking down the final moments of a cell’s life.
During aging, DNA accumulates damage, some of which can be devastating to the cell. In tests, the team found that large nucleoli had less stable rDNA than smaller ones. Also, when the structure is large, proteins and other factors that are usually excluded from the nucleolus are no longer kept out. It’s as if the nucleolus becomes leaky, letting in molecules that can wreak havoc on the fragile rDNA.
“The whole point of condensates is to separate biological reactions to help them work efficiently, but now when you have other proteins coming into the nucleolus, it leads to genome instability, which triggers the end of the lifespan,” Dr. Tyler said. These proteins can cause catastrophic problems, such as chromosomal rearrangements, to build up.
Next, the researchers plan to study nucleolar effects on aging in human stem cells. Stem cells are special because they have the potential to replace other cell types as they die. But eventually, the stem cells stop dividing, so the researchers hope to use the knowledge gained from this project to make them last longer.
Dr. Tyler and Dr. Gutierrez are excited about how different aspects of biology and aging have come together in this project, which may lead to new therapies. “I was excited that we could connect the structure of the nucleolus with the repair process in a way that could be conserved from yeast to humans,” said Dr. Gutierrez.
Source:
Journal reference:
Ignacio, G. J., & Tyler, J. K. (2024). A mortality timer based on nucleolar size triggers nucleolar integrity loss and catastrophic genomic instability. Nature Aging. doi.org/10.1038/s43587-024-00754-5.